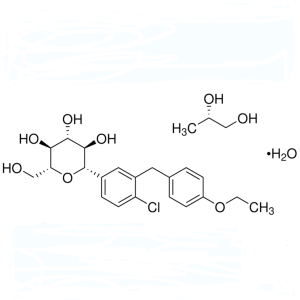
Dapagliflozin Propanediol Monohydrate CAS 960404-48-2 Assay 98.0%~102.0% API High Quality
Ruifu Chemical is the leading supplier of Dapagliflozin Propanediol Monohydrate (CAS: 960404-48-2) with high quality. Ruifu Chemical has been supplying APIs and pharmaceutical intermediates more than 15 years.
Ruifu Chemical can provide worldwide delivery, competitive price, excellent service.
Purchase Dapagliflozin Propanediol Monohydrate, please contact us by e-mail: alvin@ruifuchem.com
| Chemical Name | Dapagliflozin Propanediol Monohydrate |
| Synonyms | Dapagliflozin (2S)-1,2-Propanediol Monohydrate; Dapagliflozin Propylene Glycol Hydrate; BMS-512148 (2S)-1,2-Propanediol Hydrate |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 960404-48-2 |
| Molecular Formula | C21H25ClO6.C3H8O2.H2O |
| Molecular Weight | 502.98 g/mol |
| Melting Point | 74.0-78.0℃ |
| COA & MSDS | Available |
| Quality Standard | In-House Standard |
| Origin of Product | Shanghai, China |
| Product Categories |
API (Active Pharmaceutical Ingredient) |
| Brand | Ruifu Chemical |
| Items | Specifications | Results |
| Appearance | White to off-white powder | White powder |
| Solubility | Freely soluble in methanol, tetrahydrofuran, ethanol or Acetone, soluble in acetonitrile, Practiaclly insoluble in water |
Conforms |
| Identification HPLC | The retention time of the major peak in the chromatogram obtained with the test solution should match with that of the major peak in chromatogram obtained with standard solution in the test for assay by HPLC |
Conforms |
| Infrared Absorption | The infrared absorption spectrum of the sample shall be consistent with that of the reference substance |
Conforms |
| Halide | ≤0.02% | Conforms |
| Sulfate | ≤0.03% | Conforms |
| Organic Impurities | ||
| Any Individual Unspecified Impurity | ≤0.10% | 0.064% |
| Total Impurities | ≤0.30% | 0.083% |
| Residual Solvents | ||
| Methanol | ≤0.30% | Not Detected |
| Ethanol | ≤0.50% | Not Detected |
| Dichloromethane | ≤0.06% | Not Detected |
| Ethyl Acetate | ≤0.50% | Not Detected |
| n-Hexane | ≤0.029% | Not Detected |
| Tetrahydrofuran | ≤0.072% | Not Detected |
| Cyclohexane | ≤0.388% | 0.0026% |
| Isopropyl Acetate | ≤0.50% | 0.012% |
| Methylbenzene | ≤0.089% | Not Detected |
| Benzene | ≤0.0002% | Not Detected |
| Acetic Acid | ≤0.50% | Not Detected |
| Analysis | ||
| Propanediol | 14.0%~16.5% | 14.3% |
| Water Content (By K.F) | 3.2%-4.0% | 3.7% |
| Residue on Ignition | ≤0.10% | 0.035% |
| Heavy Metals | ≤20ppm | <20ppm |
| Assay / Analysis Method | 98.0%~102.0% of C21H25ClO6, calculated on the anhydrous, propanediol-free, and solvent-free basis | 100.4% |
| Conclusion | The product has been tested and complies with the given specifications | |
Package: Bottle, Aluminium foil bag, 25kg/cardboard drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed. Store in a cool, dry (2-8℃) and well-ventilated warehouse away from incompatible substances. Keep away from sunshine; avoid fire and heat sources; avoid moisture.
Shipping: Deliver to worldwide by air, by sea, by FedEx / DHL Express. Provide fast and reliable delivery.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Dapagliflozin Propanediol Monohydrate (CAS: 960404-48-2) was originally approved by the FDA on 8 January 2014 for use in combination with diet and exercise to improve glycemic control in adults with type 2 diabetes. It was later approved in April 2021 to reduce the risk of declining kidney function, kidney failure, cardiovascular death and hospitalisation for heart failure in adults with chronic kidney disease.
Dapagliflozin Propanediol Monohydrate is an SGLT2 inhibitor, which can be used for the treatment of diabetes.
Dapagliflozin Propanediol
C21H25ClO6 · C3H8O2 · H2O 502.99
D-Glucitol, 1,5-anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-, (1S)-, compd. with (2S)-1,2-propanediol, hydrate (1:1:1);
(1S)-1,5-Anhydro-1-C-[4-chloro-3-[(4-ethoxyphenyl) phenyl]-D-glucitol (S)-propane-1,2-diol (1:1) monohydrate;
(2S,3R,4R,5S,6R)-2-[4-Chloro-3-(4-ethoxybenzyl)phenyl]-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol
(S)-propane-1,2-diol (1:1) monohydrate
CAS RN®: 960404-48-2; UNII: 887K2391VH.
DEFINITION
Dapagliflozin Propanediol contains NLT 98.0% and NMT 102.0% of dapagliflozin (C21H25ClO6) calculated on the anhydrous, propanediol-free, and solvent-free basis.
IDENTIFICATION
• A. SPECTROSCOPIC IDENTIFICATION TESTS <197>, Infrared Spectroscopy: 197A or 197K
• B. The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• PROCEDURE
Solution A: Trifluoroacetic acid and water (0.5: 1000)
Solution B: Trifluoroacetic acid and acetonitrile (0.5: 1000)
Mobile phase: See Table 1.
Table 1
Time (min) Solution A (%) Solution B (%)
0 85 15
2 85 15
36 10 90
39 10 90
40 85 15
45 85 15
Standard solution: 0.2 mg/mL of USP Dapagliflozin
Propanediol RS in acetonitrile
Sample solution: 0.2 mg/mL of Dapagliflozin Propanediol in acetonitrile
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 220 nm
Column: 4.6-mm × 15-cm; 3.5-µm packing L1
Column temperature: 30°
Flow rate: 1 mL/min
Injection volume: 10 µL
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: 0.8–1.5
Relative standard deviation: NMT 0.85% for 6 injections
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of dapagliflozin (C21H25ClO6) in the portion of Dapagliflozin Propanediol taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of dapagliflozin from the Sample solution
rS = peak response of dapagliflozin from the Standard solution
CS = concentration of USP Dapagliflozin Propanediol RS in the Standard solution (mg/mL)
CU = concentration of Dapagliflozin Propanediol in the Sample solution (mg/mL)
Acceptance criteria: 98.0%-102.0% on the anhydrous, propanediol-free, and solvent-free basis
OTHER COMPONENTS
• CONTENT OF PROPANEDIOL
Internal standard solution: 2.64 mg/mL of USP Ethylene Glycol RS in dimethylacetamide
Standard solution: 3.0 mg/mL of USP Propylene Glycol RS in Internal standard solution
Sample solution: 20 mg/mL of Dapagliflozin Propanediol in Internal standard solution
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: GC
Detector: Flame ionization
Column: 0.32-mm × 15-m fused silica; coated with a 0.5-µm film of phase G14. [NOTE—Phases of G15, G16, G20, or G39 may also be suitable for use.]
Temperatures
Injection port: 240°
Detector: 240°
Column: See Table 2.
Table 2
Initial Temperature (°) Temperature Ramp (°/min) Final Temperature (°) Hold Time at Final Temperature (min)
150 - 150 2
150 40 240 4
Carrier gas: Helium
Flow rate: 3.5 mL/min
Injection volume: 1.0 µL
Injection type: Split, split flow 80.8 mL/min
Run time: NLT 5 times the retention time of propanediol
System suitability
Sample: Standard solution
[NOTE-The relative retention times for propanediol and ethylene glycol are 1.0 and 1.1, respectively.]
Suitability requirements
Resolution: NLT 1.5 between propanediol and ethylene glycol
Relative standard deviation: NMT 3.0% for propanediol and ethylene glycol
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of propanediol in the portion of Dapagliflozin Propanediol taken:
Result = (RU/RS) × (CS/CU) × 100
RU = peak response ratio of propanediol to ethylene glycol from the Sample solution
RS = peak response ratio of propanediol to ethylene glycol from the Standard solution
CS = concentration of USP Propylene Glycol RS in the Standard solution (mg/mL)
CU = concentration of Dapagliflozin Propanediol in the Sample solution (mg/mL)
Acceptance criteria: 14.0%-16.5%
IMPURITIES
• ORGANIC IMPURITIES
Solution A, Solution B, Mobile phase, Standard solution,
Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
Sensitivity solution: 0.1 µg/mL of USP Dapaglifloz in Propanediol RS in acetonitrile
System suitability solution: 0.2 mg/mL of USP Dapagliflozin Propanediol RS and 0.007 mg/mL of USP Dapagliflozin Related Compound A RS in acetonitrile
System suitability
Samples: Standard solution, Sensitivity solution, and System suitability solution
[NOTE-The relative retention times for dapagliflozin and dapagliflozin related compound A are 1.0 and 1.02, respectively.]
Suitability requirements
Resolution: NLT 2.0 between dapagliflozin and dapagliflozin related compound A, System suitability solution
Tailing factor: 0.8-1.5 for dapagliflozin, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Sample: Sample solution
Calculate the percentage of each individual impurity in the portion of Dapagliflozin Propanediol taken:
Result = (rU/rT) × 100
rU = peak response of each individual impurity from the Sample solution
rT = sum of all the responses from the Sample solution
Acceptance criteria: See Table 3. The reporting threshold is 0.05%.
Table 3
Name Relative Retention Time Acceptance Criteria, NMT (%)
Dapagliflozin 1.0 -
Ethyldapagliflozina 1.24 0.15
Any individual unspecified impurity - 0.10
Total impurities - 0.30
a (2S,3R,4R,5S,6R)-2-[4-Chloro-3-(4-ethoxy-3-ethylbenzyl)phenyl]-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol.
SPECIFIC TESTS
• WATER DETERMINATION á921ñ, Method I, Method Ic: 3.2%-4.0%
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in a well-closed container. Store at controlled room temperature.
• USP REFERENCE STANDARDS <11>
USP Dapagliflozin Propanediol RS
USP Dapagliflozin Related Compound A RS
(2S,3R,4R,5S,6R)-2-[4-Bromo-3-(4-ethoxybenzyl) phenyl]-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol.
C21H25BrO6 453.33
USP Ethylene Glycol RS
Ethane-1,2-diol.
C2H6O2 62.07
USP Propylene Glycol RS
Propane-1,2-diol.
C3H8O2 76.10 (USP 1-Dec-2023)